Guidelines on the Sale or Supply of Non-Prescription Medicinal Products from a Retail Pharmacy Business
The purpose of these guidelines is to facilitate compliance with the legislation in relation to the sale or supply of non-prescription medicinal products from a pharmacy.
Version 1, November 2018

Guidelines on the Sale or Supply of Non-Prescription Medicinal Products from a …
DownloadThe purpose of these guidelines is to facilitate compliance with Regulation 10 of the Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended) (S.I. No. 488 of 2008), and in addition with Regulation 5(1)(d) and 5(1)(h) in relation to the sale or supply of non-prescription medicinal products from a retail pharmacy business.
These guidelines apply to the sale or supply of non-prescription medicinal products from a retail pharmacy in all circumstances. This includes sale or supply to an individual who attends the pharmacy in person, as well as at a distance through online purchases from a pharmacy website, which is registered with the Pharmaceutical Society of Ireland (PSI) and is on the approved Internet Supply List.
Non-prescription medicinal products include medicines that have been categorised by the Health Products Regulatory Authority (1) (HPRA) as pharmacy-only medicines (which can only be sold from a pharmacy), and general sale medicines (which can be sold from non- pharmacy premises). All medicines supplied from a pharmacy, i.e. pharmacy only and general sale medicines, must be supplied by or under the personal supervision of a pharmacist. The supervision of a pharmacist is necessary to ensure the correct medicine is selected, and appropriate advice and support is provided to the patient or their representative. It also ensures that the medicine is being used to treat the correct condition and does not mask or delay the diagnosis of a more serious condition which may require immediate referral to a doctor or other healthcare professional. This provides timely access to healthcare for patients and reduces the burden on other healthcare services. Discussions with patients will often provide opportunities for health promotion or interventions and is a key opportunity for the pharmacist or trained staff member to assist and empower the patient to care for their own health.
Providing advice in the selection and supply of non-prescription medicines can be delegated in some cases to a trained staff member, but the pharmacist retains overall responsibility for the safe supply of the medicine. Therefore in order to ensure a safe service it is vital that all staff members are appropriately trained and have the required level of competence to carry out the roles and duties delegated to them, and that they know when to refer to the pharmacist for further advice.
(1) The Health Products Regulatory Authority (HPRA) is a state agency responsible for regulating medicines, medical devices and other health products.
The operation of a retail pharmacy business is governed by the Pharmacy Act 2007 and the Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended) (S.I. No. 488 of 2008). These guidelines are intended to facilitate a better understanding of, and compliance with, Regulations 5(1)(d), 5(1)(h) and 10 of the Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended), the full text of which is set out below. It should be noted that the requirement for the sale and supply of all medicinal products, including non- prescription medicines, to be carried out by or under the personal supervision of a registered pharmacist is set out in both Regulation 5(1) (d) of the Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended) and in the Pharmacy Act 2007 (2).
Regulation 5
Management and supervision of a retail pharmacy business
5. (1) The pharmacy owner and the superintendent pharmacist shall, inter alia, ensure that—
(d) the sale or supply of medicinal products, including veterinary medicinal products, and the preparation, dispensing and compounding of prescriptions, including veterinary prescriptions, at the premises, are carried out by or under the personal supervision of a registered pharmacist,
….
(h) he or she is satisfied that all of the pharmacists and other staff, employed or engaged by him or her, or under his or her management, have the requisite knowledge, skills, including language skills, and fitness to perform the work for which they are, or are to be, responsible,
Regulation 10
Counselling in the supply of medicinal products other than on foot of a prescription
10. A person carrying on a retail pharmacy business, the superintendent pharmacist and the supervising pharmacist shall ensure that, in the course of the sale or supply of a medicinal product other than on foot of a prescription and prior to such sale or supply, a registered pharmacist is satisfied that the purchaser or other such person is aware of what the appropriate use of the medicinal product is and that it is being sought for that purpose and, in so far as the registered pharmacist is aware, the product is not intended for abuse and/ or misuse.
(2) Sections 26, 27, 28 and 29 of the Pharmacy Act 2007
The statutory Code of Conduct for Pharmacists requires that pharmacists practice their profession in a manner that is directed to maintaining and improving the health, wellbeing, care and safety of patients and the public. In particular, it is expected that pharmacists use their professional judgement, skills, competence, and specialised knowledge about medicines for the benefit of patients and encourage the rational and proper use of non- prescription medicinal products. Pharmacists are required to practice autonomously and use their professional skills when making decisions regarding the sale or supply of non-prescription medicinal products, which at times may be contrary to patient expectations.
4.1 Pharmacists’ Involvement in the Sale or Supply of Non-Prescription Medicinal Products
Pharmacists’ have a legal and professional responsibility to ensure that all non- prescription medicinal products, both pharmacy-only medicinal products and general sale medicines, are supplied safely and appropriately to patients. Pharmacists are a trusted source of advice for the public on medicines and health matters in the community, in order to uphold that trust, pharmacists have a responsibility to ensure that all information provided is accurate, clinically valid and in line with current evidence and best practice. When buying medicines from a pharmacy, members of the public have the right to expect to speak to a pharmacist for advice about their healthcare needs and the pharmacist should be available in this regard.
Legislation requires that the sale or supply of all medicinal products in a pharmacy must be carried out “by or under the personal supervision of a registered pharmacist (3)”. Staff members must be aware of the legal responsibilities of the pharmacist to personally supervise the sale or supply of all medicinal products and of the professional input the pharmacist provides. Notwithstanding these legal obligations, certain tasks may be delegated to a member of staff who has been suitably trained and is working under he supervision of the pharmacist. Where this occurs, pharmacists should be satisfied that:
- staff are fully trained in the activities they are carrying out, and the information that they are providing, including the limitations of their knowledge
- staff know when it is necessary to refer to the pharmacist for additional advice, including those symptoms or signs which could indicate a serious underlying condition or disease, or medical emergency
- they are aware of consultations taking place, including the nature of any advice or guidance that is being given by the staff member
- are in a position to intervene, as may be necessary, and
- appropriate procedures are in place and utilised for the safe supply of non- prescription medicinal products in the pharmacy
Due to the nature and indication of some non- prescription medicinal products, a consultation between the pharmacist and the individual patient may be required. A pharmacist should be readily identifiable to the public at all times during the pharmacy’s opening hours. It is essential that the pharmacy owner and/or the superintendent pharmacist ensures that there is an adequate number of pharmacists and trained staff members on duty in a pharmacy to ensure the safe supply of non-prescription medicinal products alongside the operation of all other pharmacy services.
4.2 Patient Consultation
Regulation 10 (4) requires that prior to the sale or supply of all non-prescription medicinal products, a pharmacist must be satisfied that:
- the patient/purchaser is aware of the appropriate use of the medicinal product
- the medicinal product is being sought for that purpose
- the medicinal product is not intended for abuse and/or misuse
Non-prescription medicinal products may be sought either in person in a pharmacy or by online purchase from a pharmacy’s website. A request may be made for a specific medicinal product or symptoms may be described by the patient/their representative (5).
In all cases, the appropriate supply of non- prescription medicinal products is dependent on an effective consultation taking place. The effectiveness of a consultation depends strongly on the quality, nature and extent of information exchanged between the pharmacist or trained staff member, and the patient, for example through the use of open-ended questions, active listening and clear, user-friendly language which suits the needs of the patient. A good consultation is dependent not just on technical knowledge of medicinal products and health conditions, but the manner in which the consultation is carried out and the overall approach to the patient. It is important to consider both verbal and non-verbal communication (i.e. body language, eye contact, hand gestures, and tone) and take time to listen to the patient respecting the role which patients play in their own health.
Patients often have to share personal information about their medication and medical history during consultations; it is important to be aware of privacy issues and that patients may be reluctant to provide this information in the public area of the pharmacy if they can be overheard. Consultations and counselling should be provided in a discreet and respectful manner reflecting the sensitivities of the situation and individual perspective of the patient. The availability of the patient consultation area should be highlighted to patients where appropriate and a sign must be in place which informs patients that the facility exists and is available for their use. More detailed guidance on the patient consultation area can be found in the PSI’s Guidelines on Patient Consultation Areas in Retail Pharmacy Businesses. All staff members must be trained on their duty to maintain patient confidentiality and must ensure that they communicate to patients, and to each other, in such a way that patient confidentiality and privacy is maintained.
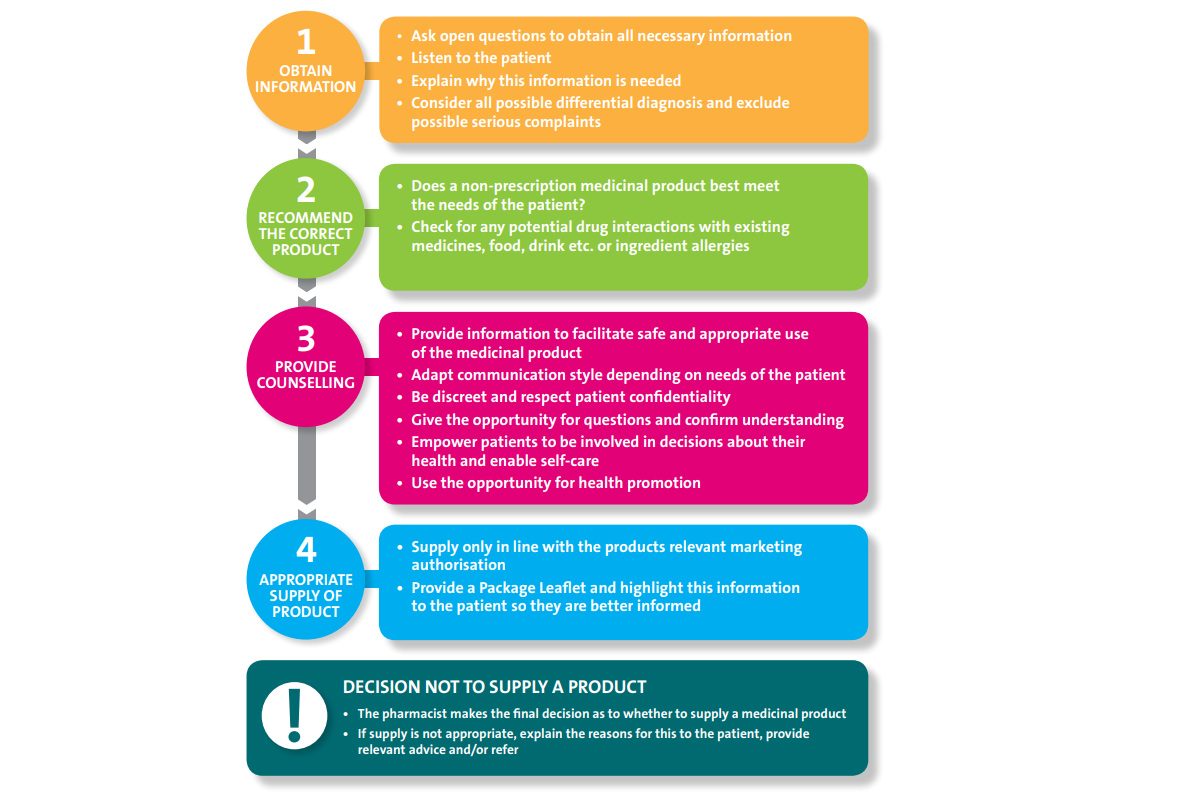
In all circumstances, consultations should have a structured approach and the steps set out below should be followed. Appendix 1 contains a summary of the key points to remember for an effective patient consultation.
4.2.1 Obtain Information
Pharmacists should be satisfied that during the course of the patient consultation, carried out by themselves or a trained staff member, all relevant information has been obtained from the patient with regard to their current condition(s), medication and lifestyle. Gathering this information enables assessment of what condition may need to be treated, possible differential diagnosis and exclusion of possible serious complaints that may need to be referred to another healthcare professional. An assessment can then be made of the most appropriate way to treat the condition,which can include recommending a medicinal product, providing health and lifestyle advice and/or signposting to another appropriate service.
Information to be obtained should include, but is not limited to:
- who is the patient
- what are the symptoms
- history of the condition or complaint
- duration of symptoms
- any relevant medical history
- any treatments or medications already tried
- any other medications/treatments being taken
Pharmacists should be mindful of how they request information. It may be useful to explain to the patient why certain information is needed, i.e. to be able to make a decision about the appropriate medicine and advice, or to recommend they need to see their doctor or another healthcare professional. It should be highlighted that if used correctly, medicines offer great benefit, but if used incorrectly medicines have the potential to do harm.
4.2.2 Recommend the Correct Product
Prior to recommending a medicinal product, it is necessary to ensure that the medicinal product best meets the needs of the patient. The information obtained from the patient should be adequate to make an assessment of medication risk factors and to evaluate the risk-benefit of making a supply of a non- prescription medicinal product. Pharmacists should be alert to any presenting signs or symptoms that may be indicative of a serious or underlying condition and require referral to another appropriate healthcare professional or service.
Screening for any potential drug interactions or ingredient allergies should be addressed, including but not limited to, interactions with other medicinal products (both prescription and non-prescription medicinal products), food, drinks, food supplements or herbal medicinal products.
Pharmacists are expected to use their professional judgement, to make the final decision as to whether making a supply of a non-prescription medicinal product is appropriate and in the best interest of the patient.
4.2.3 Provide Counselling
Effective counselling facilitates the opportunity to increase the patient’s knowledge and understanding of their health condition and any medicinal products used in the treatment of their condition as well as their treatment options. It helps to ensure the appropriate and rational use of medicinal products and reduce medication related problems, including non- adherence and unnecessary use of medicinal products. It also provides an important opportunity for health promotion advice and where possible, patients should be signposted to other available information resources, e.g. approved websites, charities or support groups.
During the course of the consultation, the pharmacist, or trained staff member, should provide the patient with all relevant information required to facilitate the safe and appropriate use of the medicinal product.
Patients should have the opportunity to ask questions and the pharmacist should confirm their understanding. When communicating information to patients, the pharmacist or trained staff member, should adapt how they present information depending on the needs of the patient, taking into account such things as their literacy, numeracy and language skills. Each consultation should be given due care and attention, as a medicine that is regularly supplied in the pharmacy may be a first request for the patient who may not be aware of the common side effects or contraindications for taking the medicine.
Counsel the patient on the following matters, as deemed appropriate:
- the name, nature and use of the medicinal product
- directions for use including dosage, dosage intervals and how to administer the medicinal product
- expected duration of treatment
- expected benefit(s) of treatment
- when improvement should be noticed
- any potential side effects considered relevant and actions to be taken should they occur
- any potential for allergic reactions
- expected outcomes of the condition or complaint
- recommended lifestyle changes
- what to do if they think the medicinal product is not working, or if symptoms change or worsen
- signs or symptoms that they should see, or discuss with, their GP or other healthcare professional immediately
- safe and appropriate storage of the medicinal product and the necessity to keep it out of reach and sight of children
- safe disposal of the medicinal product
4.2.4 Appropriate Supply of Product
Non-prescription medicinal products can only be supplied in line with their relevant marketing authorisation. The pharmacist should be familiar with the Summary of Product Characteristics (SmPC) of all medicinal products they supply, including their authorised indications, dosage, age limitations, contraindications and any special warnings. The PSI expects that with each supply of a non-prescription medicinal product a Package Leaflet (PL) is supplied to the patient.
The patient should be advised to read the information printed on the packaging and/ or the PL in order to be informed about their medicine and to get the most benefit from it. This information includes essential details such as the correct dose, instructions for use and potential side effects. Additional SmPCs and PLs of all products marketed in Ireland are available on the website of either the HPRA or European Medicines Agency (EMA).
4.2.5 Decision not to Supply a Medicine
Using their professional judgement, pharmacists make the final decision as to whether making a supply of a non-prescription medicinal product is in the best interest of the patient. If a medicinal product is deemed to be unsafe or inappropriate for a patient, the supply should not be made. In this instance, the reasons for this decision should be communicated clearly and respectfully to the patient, relevant advice should be provided and they should be referred to another healthcare professional or service as appropriate.
4.3 Additional Counselling Considerations
4.3.1 Supply to a Representative
The patient may not always attend the pharmacy in person and supply to a representative or carer may be necessary. The pharmacist or trained staff member should be satisfied that the representative is able to provide enough information to enable them to make an informed decision to ensure safe supply of a non-prescription medicinal product, and it is reasonable to make the supply in the circumstances presented. The representative should be provided with any relevant counselling for the safe use of the medicinal product to pass on to the patient. The appropriateness of the supply to a representative of the patient of any medicinal product, with a potential for dependency and/ or abuse, needs to be carefully considered by the pharmacist.
4.3.2 Medicinal Products for Infants and Children
Pharmacists and trained staff members should be particularly vigilant in the sale or supply of non-prescription medicinal products for use in infants and children as they are particularly vulnerable to overdose, side effects and are generally at greater risk of suffering harm from medication errors than adults. Supply by the pharmacist should be considered in such circumstances.
When supplying non-prescription medicinal products for an infant or child:
- check the age
- check the weight (where appropriate)
- provide accurate information regarding dose and highlight how this may change with age/weight
- demonstrate how to use any dosing equipment needed for use
- draw the parent/carer’s attention to the information in the PL provided
- advise on the storage of the medicinal product and the necessity to keep it out of reach and sight of children
When supplying medicinal products for infants or children, pharmacists should be particularly mindful of the danger of treating signs and symptoms that may be indicative of an underlying condition, which may require immediate referral. Pharmacists should also ensure that appropriate advice is provided to parents/carers on the steps to be taken should symptoms not improve or worsen, and circumstances where further healthcare professional advice should be sought.
4.3.3 Reporting Adverse Reactions
Any suspected adverse reactions to a medicinal product should be reported to the HPRA. There are several options in place for reporting suspected adverse reactions to the HPRA, as follows:
- By following the links (‘Report an Issue’ tab) to the online reporting options accessible from the HPRA website homepage (www.hpra.ie);
- Using the downloadable report form also accessible from the HPRA website, which may be completed manually and submitted to the HPRA via ‘freepost’;
Using the traditional ‘yellow card’ report, which also utilises a ‘freepost’ system. ‘Yellow cards’ are available from the HPRA Pharmacovigilance department on request; - By telephone to the HPRA Pharmacovigilance section (01 676 4971).
4.4 Internet Supply
The online supply of non-prescription medicinal products is allowed under EU and Irish law. In order for a pharmacy to supply non-prescription medicinal products based on an online order, the pharmacy must be entered on the Internet Supply List. The PSI is the designated Irish authority for supplier entry onto the approved Internet Supply List. Further information on the Internet Supply List, including its legislative basis, can be found on the PSI website.
When an online request is made for a non- prescription medicinal product, pharmacists have the same responsibility for ensuring a safe and appropriate supply of the correct medicine as if the supply was made face to face. There must be systems in place to ascertain all necessary information from the patient, a system for providing appropriate counselling (as set out in Sections 4.2.1 to 4.2.3) and for safe delivery of the medicinal product to the purchaser. Importantly, the systems should include a means of identifying inappropriate requests for medicine(s), or those that are too large or too frequent in order to ensure that medicinal products are being supplied safely.
In addition to the requirements set out above, when supplying medicines in this way the regulations (6) also require that a pharmacist must personally review each order for supply and personally supervise and authorise the supply.
The pharmacist making the supply must also check that:
- the purchaser is over 18 years old,
- the purchaser is aware that the medicinal product should be used in accordance with the recommendations for use contained in the product packaging, and
- the total quantity of the product to be supplied in the transaction is a quantity that is reasonably required for the purchasers own treatment, having regard to any previous supply to that purchaser.
Records of every online supply must be maintained in accordance with legislative requirements.
Pharmacists should carefully consider, in the course of their review, whether the patient’s needs would be best met if he/she:
- engaged with the pharmacist in a verbal review (e.g. via a telephone call or video call)
- attended the pharmacy personally, or
- was referred to another healthcare professional
For further information on internet supply, see the PSI Guidance on Internet Supply of Non-Prescription Medicines.
(3) Regulation 5(1)(d) of the Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended) and Sections 26, 27, 28 and 29 of the Pharmacy Act 2007.
(4) Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended).
(5) Hereafter the term ‘patient’ refers to individual patients and also their representative or carer.
(6)Regulation 19A(6)(C) Medicinal Products (Prescription and Control of Supply)(Amendment) Regulations 2015 (S.I. No. 87 of 2015).
Superintendent pharmacists must ensure that policies and procedures are in place to cover the appropriate sale or supply of non-prescription medicinal products by a pharmacist, or by a trained member of staff, under the personal supervision of a pharmacist. These should include procedures outlining the steps to be followed for an effective consultation and the circumstances within which referral to the pharmacist is required. Additional policies and procedures may be required for the sale or supply of non- prescription medicinal products for particular patient cohorts; these should be considered by the superintendent and supervising pharmacist in the context of all PSI guidance or guidance/information from other bodies. Examples include where non-prescription medicinal products have a potential for abuse and/or misuse or are being sought by patients in high risk groups (e.g. patients on multiple medications, pregnant women or children).
All policies and procedures should be reviewed on a regular basis and in response to errors, incidents or near misses, changes in legislation and guidance, etc. The superintendent pharmacist and supervising pharmacist must be satisfied that all pharmacists practising in the pharmacy, and all relevant staff members, are trained on, and are following, relevant and up-to date policies and procedures.
In line with Regulation 5(1)(h) (7), the pharmacy owner and the superintendent pharmacist must ensure that all pharmacists and all staff employed in the pharmacy, have all the required knowledge and skills necessary to ensure that they are capable of carrying out the work that they are responsible for. The pharmacy owner and the superintendent pharmacist should ensure that:
- all staff members are aware of their roles and responsibilities and have the appropriate competencies to carry out the roles for which they are responsible
- all staff members involved in the sale or supply of non-prescription medicinal products are appropriately trained in the pharmacy’s policies and procedures, requirements set out in the SmPC of the individual products and relevant minor ailments
- audit and review procedures are in place for staff training to ensure staff members are re-trained as needed so as to maintain up to date knowledge of non-prescription medicinal products and current advice on treating minor ailments
- all staff are made aware of their duties in relation to patient confidentiality and the consequences of breaching that duty
- staff training is documented
- all staff members are appropriately supervised and operate within the limits of their roles and responsibilities at all times
- sufficient pharmacy staffing levels are maintained to meet the level of services provided in the pharmacy
(7) Regulation of Retail Pharmacy Businesses Regulations 2008 (as amended
This self-assessment checklist is a practical tool intended to aid compliance with these guidelines and to assist superintendent and supervising pharmacists in drawing up relevant policies and procedures. While the checklist captures many important elements of the guidelines, it is not exhaustive and should only be used to assess pharmacy practice in combination with these guidelines and all other relevant guidance and requirements.
| Ask Yourself | Yes | No | N/A | Required Action |
| Are all staff members competent and trained in the roles that have been delegated to them and know when to refer to the pharmacist for advice? | ||||
| Is all relevant information provided to the patient for the safe and appropriate use of the medicine being supplied? | ||||
| Are steps taken to provide counselling in a discreet and respectful manner so that those not involved in the patients care cannot overhear? | ||||
| Is the availability of the patient consultation area highlighted to patients where appropriate, and a sign in place which informs patients that the facility exists and is available for their use? | ||||
| Is a package leaflet provided with all supplies of non-prescription medicines, and is this brought to the attention of the patient/their representative? | ||||
| Is particular care taken when medicines are supplied for infants and children, paying particular attention to selecting the correct product for their age/weight and providing appropriate counselling to the carer? | ||||
| If the pharmacist decides that it is not appropriate to supply a non-prescription medicine to the patient, based on the information provided, are the reasons for this decision clearly and respectfully explained to the patient, and are they referred to another healthcare professional or service if appropriate? | ||||
| Are the pharmacists personal supervision requirements facilitated within the pharmacy e.g through having adequate and appropriately trained staff levels on duty? | ||||
| During the pharmacy opening hours, is the pharmacist on duty readily identifiable and available to the patient? | ||||
| When an online request is made for a non-prescription medicine, are there robust systems in place to; ascertain all necessary information from the patient, provide appropriate counselling and ensure the safe delivery of the medicinal product to the purchaser? | ||||
| When an online request is made for a non-prescription medicine are there robust systems in place to identify inappropriate requests for medicines, or those that are too large or too frequent? | ||||
| Is the pharmacy owner, superintendent pharmacist and supervising pharmacist satisfied that all pharmacists in the pharmacy, and relevant staff members, are trained on, and following the policies and procedures regarding safe supply of non-prescription medicines? |
Appendix 1: Key Points for Effective Patient Consultations