Guidelines to Support Medicine Therapy Review, Counselling and Prescription Extension
These guidelines provide a principles-based outline of the responsibilities of all registered pharmacists, including those in pharmacy governance roles, regarding medicine therapy review, counselling, and extending the validity of prescriptions.
July 2024

Guidelines to Support Medicine Therapy Review, Counselling and Prescription Ext…
DownloadThese guidelines:
- Replace version 4 of the Guidelines on the Counselling and Medicine Therapy Review in the Supply of Prescribed Medicines Products from a Retail Pharmacy Business.
- Reflect the legislative change in March 2024, giving pharmacists the authority to extend the validity of certain prescriptions from a period of six months, up to a maximum period of 12 months.
- Provide a principles-based framework, which is intended to be supportive and enabling, rather than prescriptive offering flexibility for implementation.
Medicines are the most common healthcare intervention within the health system. When used correctly, medicines can prevent, cure, or alleviate disease, enhance quality of life, and improve patient outcomes.
Pharmacists play a crucial role in ensuring the safe and appropriate supply of medicines. However, this responsibility cannot be achieved in isolation; it relies on a framework of governance within the pharmacy, where those in pharmacy governance roles (pharmacy owner, superintendent pharmacist, and supervising pharmacist) establish the necessary processes and structures to support a culture of person-centred care (1) delivered by an individual pharmacist practitioner.
When reviewing medicine therapy, counselling or extending the validity of prescriptions at the end of a six-month period, pharmacists optimise patient outcomes and improve medication safety when they:
- collaborate, with each other, patients, and other healthcare professionals,
- adhere to established guidance and procedures,
- apply their professional judgement and are guided by the principles in the PSI Code of Conduct.
(1) A culture of person-centred care in pharmacy prioritises compassionate, respectful interactions, where pharmacists actively listen, tailor treatment and advice to individual needs, and empower patients with necessary information and support.
These Guidelines provide a principles-based outline of the responsibilities of all registered pharmacists, including those in pharmacy governance roles, regarding medicine therapy review, counselling and extending the validity of prescriptions (hereafter referred to as ‘prescription extension’).
The Guidelines set out six guiding principles (see Figure 1), which, when followed, support the consistent delivery of safe and high-quality person-centred care regarding medicines therapy review, counselling, and prescription extension. Pharmacists also need to consider all relevant legislation and the PSI Code of Conduct when applying these principles.
The guidelines are intended to be supportive and enabling rather than overly prescriptive, offering flexibility for implementation. This approach demonstrates confidence in pharmacists to use their knowledge, skills and expertise to make informed decisions in the interests of person-centred care.
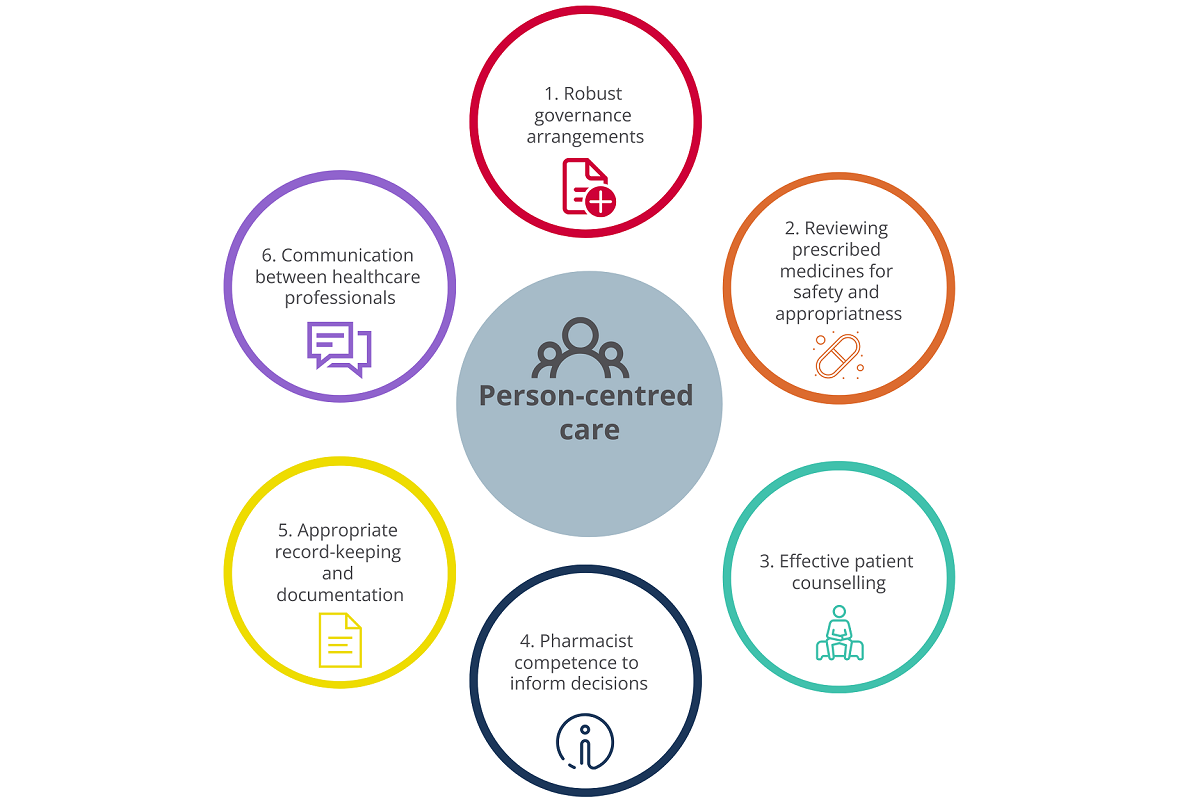
Each principle is:
- supported by a principle statement, which describes the principle,
- underpinned by concise, outcome-focused indicators that are intended to offer guidance on how adherence to each principle can be demonstrated. Additional indicators have been incorporated to clearly define the enhanced responsibilities for prescription extension.
Where ‘must’ is used in the Guidelines, this indicates an action that pharmacists or those in pharmacy governance roles are obliged to take to meet the specifications in legislation.
It is important that all relevant members of the pharmacy team are familiar with the Guidelines and understand how they are implemented in the pharmacy.
Pharmacists play an important role in reviewing prescribed medicines for both therapeutic and pharmaceutical appropriateness. This role allows them to ensure that prescribed medicines align with the patient’s health condition.
Pharmacists also play a crucial role in promoting the safe and effective supply of medicines through the patient counselling process, enabling patients to make informed decisions
about their health and wellbeing.
Legislative basis
- Regulation 9 of the Regulation of Retail Pharmacy Businesses Regulations 2008 (S.I. No. 488/2008) provides a legislative basis for the review of medicines therapy and counselling of patients in the supply of medicinal products on foot of a prescription.
Since 1 March 2024, changes to legislation enable:
- Prescribers to issue prescriptions with a legal validity of up to 12 months if they
deem it clinically appropriate. - Pharmacists to extend and dispense certain prescriptions from a period of six months up to a maximum period of 12 months where, having engaged in conversation with the patient or the patient’s representative, they determine it is safe and appropriate.
- Regulation 9A of the Regulation of Retail Pharmacy Businesses (Amendment)Regulations 2024 (S.I. No. 74/2024) provides a legislative basis for extending the validity of certain prescriptions up to 12 months.
- Medicinal Products (Prescription and Control of Supply) (Amendment) (No. 2) Regulations 2024 (S.I. No. 73/2024) provides a legislative basis to allow prescriptions to be valid for up to 12 months from the date specified on the prescription, and the associated record keeping requirements in relation to a pharmacist’s decision to extend a prescription for more than 6 months after the date specified on the prescription.
Those in pharmacy governance roles must fulfil their leadership responsibilities by working with all pharmacists practising at the pharmacy to ensure there is a clear knowledge and understanding of the legislation, and guidelines for medicine therapy review, counselling, and prescription extension,and how these are being implemented at the pharmacy.
Clear lines of responsibility and appropriate structures and processes must be in place to support a culture of safety and continuous quality improvement to ensure the sustainable provision of safe and high-quality patient care.
Indicators supporting Principle 1:
These indicators apply to those in pharmacy governance roles.
1.1 You must ensure that prior to the dispensing of each prescription and prior to the supply of the medicine, a pharmacist reviews the prescription having regard to the pharmaceutical and therapeutic appropriateness of the prescribed medicine for the patient.
1.2 You must ensure that after completing the review, a pharmacist offers counselling to patients, or their representative, on any matters they deem significant, using their professional judgement. This includes that each patient receives sufficient information and advice for the correct use and storage of the prescribed medicine.
1.3 You ensure that clear, structured documented procedures are developed and implemented in the pharmacy for medicine therapy review, counselling, and prescription extension. It is important that procedures accurately reflect legislation and these Guidelines and are specific to the practice at the pharmacy.
1.4 You monitor and audit the implementation of the documented procedures at appropriate intervals to ensure they continue to be fit for purpose, and to check there is compliance with the legislation and these Guidelines. It is important that any identified areas for improvement are actioned and monitored as part of a commitment to continuous quality improvement.
1.5 You support staff training and professional development to improve skills, knowledge and understanding of roles and responsibilities related to medicine therapy review, counselling, and prescription extension.
1.6 You provide access to an appropriate evidence base to support the safe and rational use of medicines, such as reference books and/or online resources on medicines, drug interaction alert functionality as part of the computer dispensing system, and appropriate national prescribing/clinical guidelines.
1.7 You provide the necessary staffing levels (including pharmacist cover), a fit-for purpose physical pharmacy environment, and equipment needed for the safe and appropriate supply of prescribed medicines from the pharmacy, in line with legislation and guidance.
1.8 You support and empower pharmacists in the pharmacy to use their professional autonomy (2)and judgement to provide the right care and support, at the right time, to ensure the health, safety and wellbeing of patients and the public. You don’t use targets or incentives, which could adversely influence decisions affecting patient care.
1.9 You support an open and honest safety culture (3) in the pharmacy with a focus on learning for improvement.
Additional indicator that applies to prescription extension
1.10 Prior to extending the validity of a prescription, you must ensure that a pharmacist reviews the prescription for suitability and appropriateness, considering individualised patient circumstances and having engaged in a conversation with the patient or their representative.
(2) Professional autonomy refers to the right and ability of pharmacists to make independent decisions within their scope of practice, guided by the Code of Conduct and patient needs.
(3) A safety culture exists when issues relating to patient safety and staff are critically reviewed and discussed within a pharmacy team and a culture which supports open disclosure is in place.
Pharmacists must review each prescription, using their knowledge, skills, and competence to evaluate the safety, appropriateness, and effectiveness of the prescribed medicine.
Pharmacist- medicine reviews play an important role in promoting safe, effective, and person-centred care.
Indicators supporting Principle 2:
These indicators apply to all pharmacists.
2.1 You verify that all necessary and relevant information to which you have access is considered when evaluating the appropriateness of each prescription.
2.2 You must review and evaluate each prescription for suitability, safety, and appropriateness of the medicine for the patient, using your professional judgement and considering individual patient circumstances. At a minimum, the review must include screening for any potential therapy related issues associated with the prescribed medicine, which you are aware of, or may become aware of during your practice (see Figure 2).
2.3 You take measures to stay informed and update your knowledge regarding changes in information on medicines, new medicines and clinical guidelines, as part of your ongoing professional development.
Additional indicators that apply to Prescription Extension
2.4 You review and evaluate the existing prescription for suitability, safety, and appropriateness, considering individual patient circumstances. The prescription must not be extended where the prescriber includes the instruction “do not extend” on the prescription.
2.5 Having reviewed the prescription, you are satisfied that, having regard to the pharmaceutical and therapeutic appropriateness of the prescribed medicine, you can safely continue to dispense the prescription beyond six months after the date on the prescription. This must involve a conversation with the patient or their representative and be guided by person centred criteria (see Figure 3). The prescription must not be dispensed more than 12 months after the date specified on the prescription.
Figure 2: Potential therapy-related issues
Potential therapy-related issues to be screened for includes those which may be due to:
- Therapeutic duplication
- Interactions with other medicinal products (including serious interactions with non-prescription medicinal products, herbal products, or foods)
- Incorrect dosage or duration of treatment
- Allergic reactions
- Clinical abuse and/or misuse
Figure 3: Person centred-criteria
Person-centred criteria to consider as part of the patient conversation:
- Is there a continuing need or chronic condition?
- Are there any signs or symptoms indicative of a possible adverse reaction?
- Has the history with the prescribed medicine changed?
- Is the decision to extend in compliance with proper patient care and in the patient’s best interest?
Additional points to consider for prescription extension:
- A prescription for certain medicinal products must not be extended, for example, a Controlled Drug specified in Schedule 2, 3 or 4 to the Misuse of Drugs Regulations 2017 (S.I. No. 173 of 2017)
- Other medicinal products may be excluded from prescription extension for operational and reimbursement reasons. It is therefore important that pharmacists remain up to date with these exclusions.
Pharmacists have a professional and legal obligation to provide patient counselling when supplying prescribed medicines, whether in person with a patient or their representative, or using technology to communicate at a distance. It is essential that patients understand how to use their medicines correctly, including dosage instructions, potential side effects and any special precautions.
Actively involving the patient as an equal partner in the patient counselling process enables patients to make informed decisions that are right for them in respect of managing their own health. The information provided should be individualised.
Indicators supporting Principle 3:
These indicators apply to all pharmacists.
3.1 You must ensure that the patient, or their representative, has sufficient information and advice for the correct use, storage, and disposal of prescribed medicines. Any information provided to patients should be evidence-based, objective and accurate and tailored to the patient.
3.2 You must offer to counsel patients or their representative on any matters you deem significant, using your professional judgement. This should apply every time a
prescription is dispensed and should not be limited to new prescriptions or new medicines.
Information to consider discussing as part of the counselling process:
- What the medicine is and why it has been prescribed.
- The directions for use. It is important that extra care is taken to ensure that the directions for use are explained to parents/guardians of children.
- The therapeutic benefit which may be expected from the use of the medicine.
- Likely side effects and how to manage them.
- Any special precautions to be taken when using the medicine.
- The importance of the need for compliance with the directions for use.
- Where a medicinal product has been interchanged in line with prescriber’s instruction/consent, or in accordance with the HPRA interchangeable list.
- What to do if a patient misses a dose.
- The correct use of therapeutic aids and medical devices (including demonstration of these, if applicable)
- Information relating to storage requirements, expiry dates, safe disposal.
- Information relating to monitoring requirements, where appropriate.
- Any other matters which may be included or referred to in the Summary of Product Characteristics (SmPC) for the medicinal product concerned. You should also encourage the patient/carer to read the Patient Information Leaflet themselves and if they have any queries to contact a pharmacist or their doctor.
The points above are not exhaustive, and you may wish to consider additional factors during the counselling process.
3.3 You endeavour to address any communication challenges and adapt your communication style or methods to meet the needs of each individual patient so that they are facilitated to make informed decisions and choices. If necessary, consider ways of making information accessible and understandable, for example, use pictures, symbols, large print, different language, or digital health tools, where appropriate.
3.4 You ensure patient dignity, confidentiality and privacy are respected during counselling. This applies whether counselling occurs in person, through a patient’s representative or via remote communication technology. Any method of communication (including communication by digital electronic means) used in the provision of counselling to patients must be in compliance with the appropriate Data Protection legislation. (4)
3.5 You undertake ‘in person’ counselling in an area of the pharmacy, such as the patient consultation area, that can support patient confidentiality and encourage patient involvement and understanding. Additional indicators that apply to prescription extension
3.6 You provide the patient with all relevant information in a way they can understand,
including the rationale for your decision and any monitoring or follow-up required.
3.7 You must inform the patient that the decision to extend will be shared with their prescriber.
3.8 In circumstances where you decide not to extend the prescription, you provide the patient with the rationale for this decision, explore emergency supply options if suitable, and refer the patient back to their prescriber, as appropriate.
(4) Further information on Data Protection is available on the Data Protection Commission Website www.dataprotection.ie.
Any decision made to supply prescribed medicine should be within a pharmacist’s expertise and within the scope of their knowledge, skills, and clinical competence.
Pharmacists are responsible and accountable for their decisions in medicine therapy review, counselling, and prescription extension.
Fulfilling this responsibility is conducted in partnership with the patient to ensure the safe and appropriate supply and use of prescribed medicines.
Indicators supporting Principle 4:
These indicators apply to all pharmacists.
4.1 You follow the PSI Code of Conduct, practice in accordance with legislation and guidance and place person-centred care at the centre of your decisions.
4.2 You use your knowledge, skills, and competence, in partnership with the patient, to ensure the prescribed medicine is safe and appropriate. Assessing patient circumstances involves evaluating risks and benefits based on the pharmacist’s knowledge and understanding of the patient’s condition and of the prescribed medicines.
Additional indicators that apply to prescription extension
4.3 Before deciding to extend a prescription, you are satisfied that you have sufficient expertise, clinical competence, knowledge, and understanding of the patient’s condition, and the prescribed medicine, along with any other relevant information.
4.4 You are responsible and accountable for your decision making when extending the validity period of a prescription. For subsequent supplies, pharmacists must continue to assess the pharmaceutical and therapeutic appropriateness of the prescribed medicine, and they may choose not to dispense it, even if a decision to extend had been previously made. This ensures that patient safety remains paramount, and decisions are based on the most current review of the patient’s needs.
The keeping of accurate records which clearly show when prescribed medicines are supplied and to whom, together with any professional advice provided, is a key requirement for establishing accountability and responsibility.
The keeping of appropriate records also supports the provision of safe services, continuity of care, evidence-based healthcare, good professional practice, and appropriate sourcing of medicines.
Indicators supporting Principle 5:
These indicators apply to all pharmacists.
5.1 In addition to fulfilling the legal requirements concerning record-keeping for the supply of prescribed medicines, you record any professional advice or services provided to the patient in the patient medication record. This documentation will help demonstrate the rationale for your actions and may encompass various aspects such as collaborations with other healthcare professionals, patient counselling, any intervention made and any relevant follow-up conversations with the patient. It is important that this information is recorded in a clear, concise, and consistent manner to ensure others working in the pharmacy can easily understand the information.
Additional indicators that apply to prescription extension
5.2 You must keep a record in relation to the decision to extend the prescription in the
register. (5) The record should clearly and contemporaneously document the reasons and patient-specific details that supported the decision, while also including certain information specified in legislation (6);
a) date of the decision to extend,
b) the name and PSI registration number of the registered pharmacist who made the decision to extend,
c) details of the review undertaken, and the decision made by the pharmacist, and
d) the method used to notify the prescriber (see Principle 6: Indicator 6.2).
5.3 You must keep all records in the pharmacy in accordance with pharmacy record-keeping requirements.
5.4 In circumstances where you decide not to extend the prescription, you record the rationale for this decision in the patient medication record.
(5) Regulation 10(1)(a) of the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as
amended). This register is often referred to as the ‘prescription register’, ‘daily dispensing report’, ‘daily audit’
or ‘daily print-out.
(6) Regulation 10E of the Medicinal Products (Prescription and Control of Supply) (Amendment) (No. 2)
Regulations 2024. The purpose of these Regulations is to provide for the circumstances in which prescriptions
may be dispensed more than 6 months after the date specified on the prescription.
Regular and open communication between pharmacists and other healthcare professionals is important as it ensures that all members of the healthcare team are informed and aligned in their approach to patient treatment and care.
Effective communication promotes continuity of care, improves safety, and enhances patient outcomes.
Indicators supporting Principle 6:
These indicators apply to all pharmacists.
6.1 You raise any concerns identified during the review of a prescription with the prescriber. As part of this interaction, you should clearly highlight the issues identified and, where relevant, be forthcoming with advice or suggestions on appropriate solutions based on your expertise and knowledge. Any changes should be clearly communicated to the patient and documented.
Additional indicators that apply to prescription extension
6.2 If you extend the validity of a prescription, you must make reasonable efforts to notify the person who prescribed the medicinal product, or another relevant primary healthcare professional, of the decision to extend. This notification must be given within seven days following the decision.
PSI Information
For more information and resources to support medicine therapy review, counselling and prescription extension please see the PSI website.
Please also refer to our Guidance on Pharmacy Governance roles where we set out the responsibilities of the pharmacy owner, superintendent pharmacist, supervising pharmacist and all pharmacists involved in the operation of a retail pharmacy business.
Irish Institute of Pharmacy (IIOP)
The IIOP offers a range of information and resources for pharmacists, including continuing professional development (CPD) opportunities, publications, and events. Please refer to the IIOP website for training programmes, information events, and training information that may support you in your practice regarding medicine therapy review, counselling, and prescription extension.
HSE PCRS
The HSE PCRS provides pharmacists and pharmacies with various information and resources, including reimbursement details.
- In addition to the Guidelines, pharmacists can also refer to a set of frequently asked questions on prescription extension as an additional support resource.
- We also have information for Pharmacists on the Expert Taskforce

