Safe Prescribing and Dispensing of Controlled Drugs: Medical Council/PSI Joint Guidance
This joint guidance aims to facilitate safer prescribing and dispensing of controlled drugs (CDs), with a particular focus on controlled drugs in schedule 2, 3 and schedule 4 part 1.
Version 3, April 2025

Safe Prescribing and Dispensing of Controlled Drugs
DownloadThis resource aims to facilitate safer prescribing and dispensing of controlled drugs (CDs), with a particular focus on controlled drugs in schedule 2, 3 and schedule 4 part 1. It should be used by all prescribers and pharmacists in the collaborative, safe and effective care of patients. This guidance was first produced in 2017 and has been updated to reflect further
legislative changes.
While this guidance provides some information on legal requirements applicable to hospital or residential settings, it is primarily aimed at professionals working in a primary care setting.
Controlled Drugs Legislative Change
This joint guidance was first produced in 2017 following changes made by the Misuse of Drugs Regulations 2017. The guidance was updated in 2024 to reflect changes introduced by amendments to the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended), including changes relating to the emergency supply of schedule 2, 3 and 4 controlled drugs. Additionally, the guidance was updated in light of changes introduced by the Medicinal Products (Prescription and Control of Supply) (Amendment) Regulations 2020. These changes introduced the electronic transmission of prescriptions, including those for controlled drugs, through the national electronic prescription transfer system, and established the legal requirements pertaining to prescriptions sent and received in this way.
What is a Controlled Drug and what are Schedules?
Substances, products, or preparations, including certain medicines, that are either known to be, or have the potential to be, dangerous or harmful to human health, including being liable to misuse or cause social harm, are subject to control under the Misuse of Drugs Acts 1977 to 2016. They are known as “controlled drugs”.
The Misuse of Drugs Regulations categorise controlled drug substances into five schedules (ranging from the most tightly controlled in schedule 1 to the least tightly controlled in schedule 5). Schedule 4 is divided into part 1 and part 2. The controlled drugs in each of these schedules, in practice and in this document, may be referred to as CD1s, CD2s, CD3s, CD4 part 1s, CD4 part 2s and CD5s, respectively.
Each schedule contains various drug substances and drug products based on their perceived medical benefit and their risk to public health. There are different restrictions to control the supply of each schedule of controlled drugs. CD1s have the most restrictions and CD5s have the least restrictions. Some CD5s are available to patients without a prescription.
In this guidance we focus primarily on the controlled drugs found in schedule 2, schedule 3 and schedule 4 part 1. The supply of these controlled drugs is subject to more specific requirements than those in schedule 4 part 2 and schedule 5.
We do not cover Cannabis for medical use, classified as specified controlled drugs, however guidance on the Medicinal Cannabis Access programme is available from the Department of Health (1). We also do not specifically refer to other schedule 1 controlled drugs as they are not commonly prescribed or supplied and are not regarded as having any therapeutic purposes. They may only be prescribed subject to Ministerial Licence.
| Schedule | Examples of Controlled Drugs in Each Schedule |
| Schedule 1 | Substances not ordinarily used as medicines for example, Raw Opium, Coca Leaf |
| Schedule 2 | Opiate substances for example, Morphine, Fentanyl and Oxycodone Some Stimulants for example, Lisdexamphetamine |
| Schedule 3 | Certain Benzodiazepines and painkillers for example, Temazepam, Flunitrazepam, Pentazocine, Ketamine |
| Schedule 4 Part 1 | Most Benzodiazepines and ‘Z-drugs’ for example, Diazepam, Alprazolam, Clonazepam, Midazolam and Zolpidem |
| Schedule 4 Part 2 | Certain Anti-Epileptics for example, Phenobarbitone <100mg Certain MAOIs for example, Selegiline |
| Schedule 5 | Lower strengths of painkillers for example, Codeine (below specified concentration) |
Please note: The above table contains examples only and is not an exhaustive list.
The schedules are set out in the Misuse of Drugs Regulations 2017 (as amended), and changes such as additions or reclassifications are made by way of amendments to the legislation. All prescribers and pharmacists must familiarise themselves with, and refer to, the complete lists of controlled drugs contained in each schedule.
The Health Products Regulatory Authority (HPRA) website should be checked for accurate and up-to-date information regarding the classification of authorised medicines containing controlled drugs i.e. schedule 2, schedule 3, schedule 4 part 1, schedule 4 part 2 and schedule 5. Please see Appendix 6 for more information on how to carry out this search.
The HPRA is contactable on +353 1 676 4971 or at info@hpra.ie.
Prescriptions for controlled drugs can be written privately or on a “Health prescription” which is also referred to a to as a GMS (General Medical Services) prescription. Prescriptions can be paper-based or transferred through the national electronic prescription transfer system, also referred to as Healthmail. Healthmail is the system approved by the Health Service Executive (HSE) for the transfer of prescriptions, in a permanent and unalterable form, by electronic means. It was established following amendments to legislation in 2020 (2). Further information on Healthmail is available at www.healthmail.ie.
Where a Schedule 2 or 3 Controlled Drug prescription is transferred through Healthmail, the specific prescription writing requirements still apply, however, these do not need to be in the prescriber’s own handwriting. As a prescription sent through Healthmail is traceable back to the prescriber, a signature is not required on the prescription. However, the pharmacist must still be satisfied that in their professional judgement it is safe to make any supply in the context of the information received. Examples of controlled drug prescriptions and a reference table are provided in this guidance.
“Medical practitioner” in this guidance refers specifically to medical doctors registered in Ireland. “Prescribers” in this guidance refers to healthcare professionals with prescriptive authority for controlled drugs i.e. doctors, nurse prescribers, dentists, veterinary practitioners, and midwives, who are registered in Ireland.
Note: A prescription for a controlled drug which has been prescribed by a practitioner or prescriber (with status equivalent to an Irish practitioner or prescriber) in another EEA state or the UK is not valid for dispensing in Ireland. (3)
A “repeat prescription”, as defined in the Medicinal Products (Prescription and Control of Supply) Regulations 2003, as amended, means a prescription which may be dispensed more than once.
Schedule 2 and schedule 3 controlled drugs cannot be repeated.
Schedule 4 (part 1 ) controlled drugs may be repeated in line with the directions of the prescriber up to a maximum of six months.
Schedule 4 (part 2) and schedule 5 controlled drugs may be repeated in line with the directions of the prescriber.
Pharmacists are required to use their professional judgement and decision making regarding every occasion on which a prescription is repeated, and must ensure the therapeutic appropriateness of the supply, taking account of the specific patient needs. Further guidance on the role of pharmacists in assessing the validity of prescriptions and the appropriateness of repeating prescriptions will be made available by PSI.
“Instalments” allow the total quantity of the medicine prescribed to be dispensed in smaller, specified amounts, at specified intervals.
All controlled drugs can be legally dispensed in this manner. However, in accordance with the Misuse of Drugs Regulations 2017 (as amended), ‘the number of instalments and the intervals at which the instalments may be dispensed’ must be specified on prescriptions for schedule 2, schedule 3 and schedule 4 part 1 prescriptions. In the case of schedule 2 and schedule 3 prescriptions, the first instalment must be dispensed within 14 days of the date stated on the prescription, and no instalments may be issued later than 2 months after this date. Please see Appendix 1a for an example of correctly specified instalment directions.
A pharmacist can provide an emergency supply of a prescription only medicine based on a request from a prescriber (registered medical practitioner, registered dentist, or registered nurse prescriber). This is allowable in emergency situations where the prescriber is unable to supply the prescription immediately but undertakes to provide it within 72 hours.
A pharmacist can provide an emergency supply of a prescription only medicine based on a patient’s request, where they have interviewed the patient and are satisfied that there is an
immediate need for the supply, it is impracticable to obtain a prescription without undue delay, the treatment has previously been prescribed for the patient (by a medical practitioner, registered dentist, or registered nurse prescriber), and the appropriate dose can be verified.
Pharmacists are only permitted to provide emergency supplies of controlled drugs in schedule 2, 3 or 4 whether at the request of a prescriber or a patient, when further specific conditions are met in accordance with the MedicinalProducts (Prescription and Control of Supply) Regulations 2003 (as amended)4, when in the pharmacist’s opinion –
- there is an immediate need for the supply of the controlled drug to be made,
- it is safe, appropriate, and necessary to make the supply for the continued treatment of the patient,
- it is not possible to obtain a prescription without undue delay,
- the treatment has previously been prescribed for the patient, and
- no more than 5 days treatment or the nearest equivalent minimum dosage pack unit size is being supplied,
and additionally in the case of a request by a patient
- an emergency supply of the controlled drug has not already been supplied based on a request by the patient since their last supply on foot of a prescription.
Note: Pharmacists can provide an emergency supply of methylphenobarbitone, phenobarbitone, phenobarbitone sodium, midazolam, clobazam, or clonazepam, at the request of either a prescriber or a patient, without having to satisfy the additional specific conditions above, where it is for the treatment of epilepsy.
There is a strict system of control in place, both nationally and internationally, around the movement and supply of controlled drugs5. These controls are intended to enable safe access to these medicines in light of the serious nature of the drugs concerned and substantial potential for abuse and misuse of these medicines.
In Ireland, these medicines are controlled by the Misuse of Drugs Acts and Regulations (3). These controls include restrictions on the people who can obtain or possess controlled drugs, and the strict legal obligations placed on pharmacists and prescribers charged with responsibility for the safe control of these substances.
The Misuse of Drugs Regulations 2017 (as amended) are statutory instruments which are vital in protecting patient health and safety. Failure to adhere to this legislation may result in patients receiving inadequate care and unnecessary and unacceptable burden and stress.
All prescribers (including doctors) and pharmacists must adhere to these regulations. It is an offence not to adhere to these regulations.
Additionally, medical practitioners and pharmacists, when registering with the Medical Council and the Pharmaceutical Society of Ireland (PSI) respectively, commit to adhering to professional standards and ethical guidelines set by their regulator.
Medical practitioners adhere to the Medical Council's Guide to Professional Conduct and Ethics for Registered Medical Practitioners, which explicitly states that they must prescribe in compliance with the Misuse of Drugs legislation (6).
Pharmacists must adhere to the PSI's Code of Conduct- Professional Principles, Standards and Ethics for Pharmacists, which states that pharmacists must ‘make ethical decisions, while observing relevant legislation, practice standards and guidance’. (7)
Any patient requiring a controlled drug for their treatment is entitled to a valid prescription, whether it be in paper or transferred through Healthmail. It is an offence for medical practitioners to write or produce a controlled drug prescription that doesn’t comply with the legislative requirements, and it is an offence for a pharmacist to supply controlled drugs from a prescription unless it meets the requirements. It is therefore incumbent upon both medical practitioners and pharmacists to ensure they are aware of their responsibilities and obligations under the Misuse of Drugs legislation to ensure the safe and appropriate supply of controlled drugs to their patients.
- Provide a valid prescription which meets the requirements of the legislation (6)
- Be satisfied as to the identity of the person for whose treatment the prescription is to be issued
- Follow relevant national and international prescribing guidelines
- Within reason, be available to confirm or discuss any matters related to the prescription and the patient
- Ensure the safe keeping of prescription pads to reduce the risk of theft and forgery
- Facilitate appropriate withdrawal of controlled drugs and follow-up and refer as necessary
- Only supply controlled drugs based on a legally valid prescription (4) (be it paper or transferred via Healthmail), or, by way of an essential and legally compliant emergency supply.
- Be satisfied that the signature of the prescriber is genuine, and in the case of a prescription transferred by Healthmail, that it contains the professional registration number of the person issuing it, and can be traced back to the prescriber.
- Prior to supply, be satisfied as to the identity of the person or bona fide representative presenting the prescription or collecting controlled drugs.(8)
- Be vigilant for forgeries or unusual prescribing patterns.
- Store controlled drugs in a safe manner, in accordance with the relevant legislation.(9)
- Record all supplies of schedule 2 controlled drugs (CD2) in the pharmacy’s controlled drugs
register.(10) - Communicate with the medical practitioner if there is any query about the prescription, or care of the patient.
- Adhere to national guidelines and facilitate appropriate withdrawal of controlled drugs.
Ensure follow-up and referral to other healthcare professionals/services as necessary.
Note: The figure below refers to paper-based prescriptions for schedule 2 and 3 controlled drugs. Where schedule 2 and 3 controlled drug prescriptions are transferred via Healthmail, it is not necessary for the particulars indicated below to be in the prescriber’s own handwriting. Prescriptions sent by Healthmail do not need to include the prescriber’s signature in so far as they can be traced electronically and securely back to the issuing practitioner.
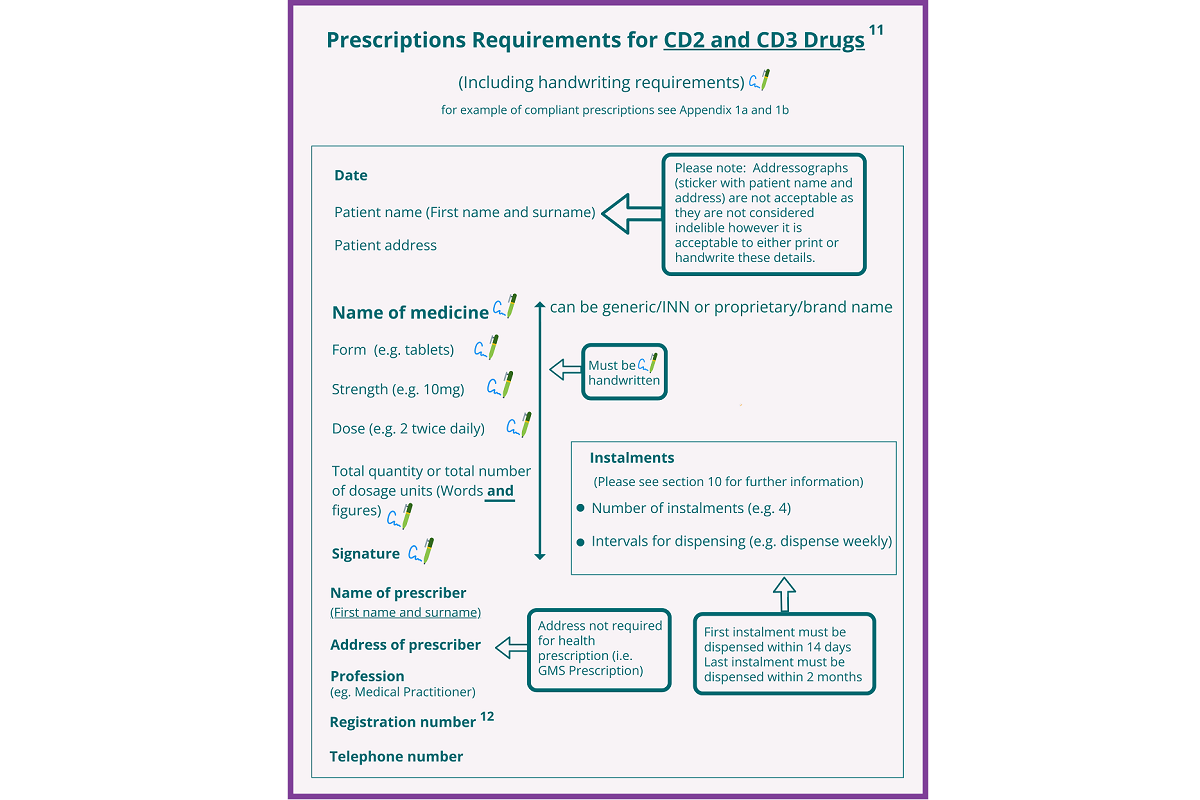

Prescriptions Required for CD2 and CD3 Drugs
The prescription requirements for opioid substitution prescriptions and schedule 4 part 1 CD prescriptions are shown in the box below.
Paper-based prescriptions must be signed by the prescriber in his or her usual signature.
Healthmail prescriptions for opioid substitution or schedule 4 part 1 CDs do not need to include the prescriber’s signature in ink in so far as the prescription can be traced electronically and securely back to the issuing practitioner.
Note: Opioid Substitution Treatment Prescription Forms are a specific type of prescription form and are the only form which can be used for prescribing methadone or buprenorphine for opioid substitution therapy. Opioid substitution prescription forms can be written and signed by the prescriber, and physically presented in the pharmacy, or they may be scanned and forwarded to the pharmacy through Healthmail. Where Healthmail is used to transfer the prescription, there is no requirement for a paper copy to be posted to the pharmacy also.
Note: An example of a schedule 4 part 1 private prescription, a schedule 4 part 1 GMS repeat prescription and a prescription for methadone are included in Appendix 2, 4 and 5.


Prescription Validity and Instalments
Prescriptions are valid for up to twelve months from the date of issue depending on the drug and the prescriber’s instruction. Pharmacists are able to exercise professional judgement on extending the period of validity of prescriptions in certain circumstances from six months up to a maximum of 12 months. A prescriber may also indicate on a prescription that it is not to be extended. As highlighted earlier, schedule 2 and schedule 3 controlled drugs cannot be repeated.
Schedule 4 (part 1) controlled drugs may be repeated in line with the directions of the prescriber up to a maximum of six months. Prescriptions for schedule 4 (part 1) medicines cannot be extended by a pharmacist.
There are specific restrictions on the period of validity of controlled drug prescriptions:
- The first instalment or supply on prescriptions for CD2 or CD3 drugs cannot be dispensed before the date of the prescription or later than 14 days after that date.
- Unless it is a GMS prescription, a prescription for a CD2, CD3 or CD4 part 1 drug must include the address of the prescriber (address must be within Ireland).
- Repeat prescriptions are not permitted in the case of CD2 or CD3 drugs.
- Prescriptions for CD2, CD3 and CD4 part 1 may be dispensed in instalments, provided that the intervals between instalments and the number of the instalments are specified.
- For CD2 and CD3 the first instalment must be dispensed within 14 days and the last instalment must be dispensed no later than two months from the date on the prescription.
- Emergency supplies of CD2, CD3 or CD4 drugs, are permitted in limited and specific circumstances, and where they comply with the legislation. (12)
- Emergency supplies of methylphenobarbitone, phenobarbitone, phenobarbitone sodium, midazolam, clobazam and clonazepam, are permitted where they comply with the legislation and are for the treatment of epilepsy.(4)
For ease of reference, Appendix 7 contains a table which clearly shows the prescription requirements for schedule 2, schedule 3 and schedule 4 part 1 controlled drugs.
Requisitions (15)
Requisitions for supplies of controlled drugs can be written privately or on a “health service requisition” form, by persons authorised under the legislation. Requisitions for controlled drugs must include:
- Name, address, and profession of the person requesting
- Signature of the person requesting Date of requisition
- Registration number of the person requesting (12)
- Name of the controlled drug
- Purpose of supply
- Total quantity of the medicine(s) to be supplied
The medical practitioner obtaining controlled drugs through the use of a requisition should be asked by the pharmacist to produce identification.
An example of a requisition can be found in Appendix 3.
12.1 Communication
Good communication is essential to the effective functioning of healthcare teams (6),(7). The existence of good, open communication channels between pharmacists and prescribers is of particular importance in assuring the safe and
efficient supply of controlled drugs to patients. By having in place a strong system of partnership, firmly based in the shared care of patients, efficiencies can be achieved in assuring that patients receive the best possible care in a more integrated system. These links are well established. In prescribing and dispensing controlled drugs, where there is any doubt or confusion around the prescription, dosage, supply and administration, pharmacists and medical practitioners should engage with each other without delay. This is particularlyimportant at transitions of care and where the initial prescriber is not their usual GP, they should have clear communication with the patient’s GP and the pharmacist, as necessary.
12.2 Identifying Risk Factors/High Risk Patients
As part of this collaborative relationship, pharmacists and prescribers should, in particular, communicate and collaborate in the care of patients in high-risk groups.
Examples include:
- Patients with drug dependency issues
- Patients receiving addiction treatment services
- Patients under the management of multiple doctors
- Patient population who attend pain clinics
- Patients transitioning from one place of care to another
- Patients in residential care settings
- Patients at risk of developing sleep disordered breathing
- Patients in the prison system
- Patients with mental health issues
- Patients living complex lives due to factors such as homelessness
- Patients with additional care requirements
- Patients with poor health literacy or patients who don’t have English as their first language
- Paediatric patients
- Older people
- Pregnant/breast feeding women
- Patients with physical/intellectual disabilities
- Patients suffering from chronic illnesses
- Patients receiving palliative care
Engagement within and between the professions in the shared care of patients in these groups is particularly important to meet patients’ care needs. This includes shared responsibility for follow up and after-care for all patients, and collaborative working in the implementation of appropriate withdrawal procedures for controlled drugs.
12.3 Relevant National Resources and Professional and Clinical Guidance
It is essential in the prescribing, dispensing and supply of controlled drugs that pharmacists and prescribers are aware of and have access to national resources, and adhere to current national and international guidelines. For example:
- The Health Products Regulatory Authority (HPRA) website for the confirmation of the scheduling of medicines containing controlled drugs (e.g. is a medicine a CD2 or CD3) (See Appendix 6)
- The online registration databases of both the Medical Council and the PSI: to confirm registration, find contact details and be aware of any conditions attached to the registration of healthcare professionals
- Resources or advice from relevant healthcare regulators, including the Medical Council, the PSI and the Health Information and Quality Authority (HIQA)
- National Clinical Guidelines from the National Clinical Effectiveness Committee and relevant faculties
- HSE Clinical Guidelines for Opioid Substitution Treatment (OST)
- HSE Medicines Management Programme Guidance on appropriate prescribing of Benzodiazepines and Z-drugs (BZRA) in the treatment of anxiety and insomnia
- Relevant international Guidelines from bodies such as the National Institute for Health and Care Excellence (NICE)
- All relevant legislation is available at www.irishstatutebook.ie
The above list is for ease of reference only and should not be considered exhaustive.
This guide is not a legal document. Its intention is to facilitate prescribers and pharmacists in interpreting several pieces of legislation and provide a practical tool in the collaborative care of patients. Links to relevant legislation have been provided for direct consultation.
Note: This example refers to a paper-based prescription for a schedule 2 controlled drug. Where schedule 2 and 3 controlled drug prescriptions are transferred via Healthmail, it is not necessary for the particulars indicated below to be in the prescriber’s own handwriting. Prescriptions sent by Healthmail do not need to include the prescriber’s signature in ink in so far as they can be traced electronically and securely back to the issuing practitioner.
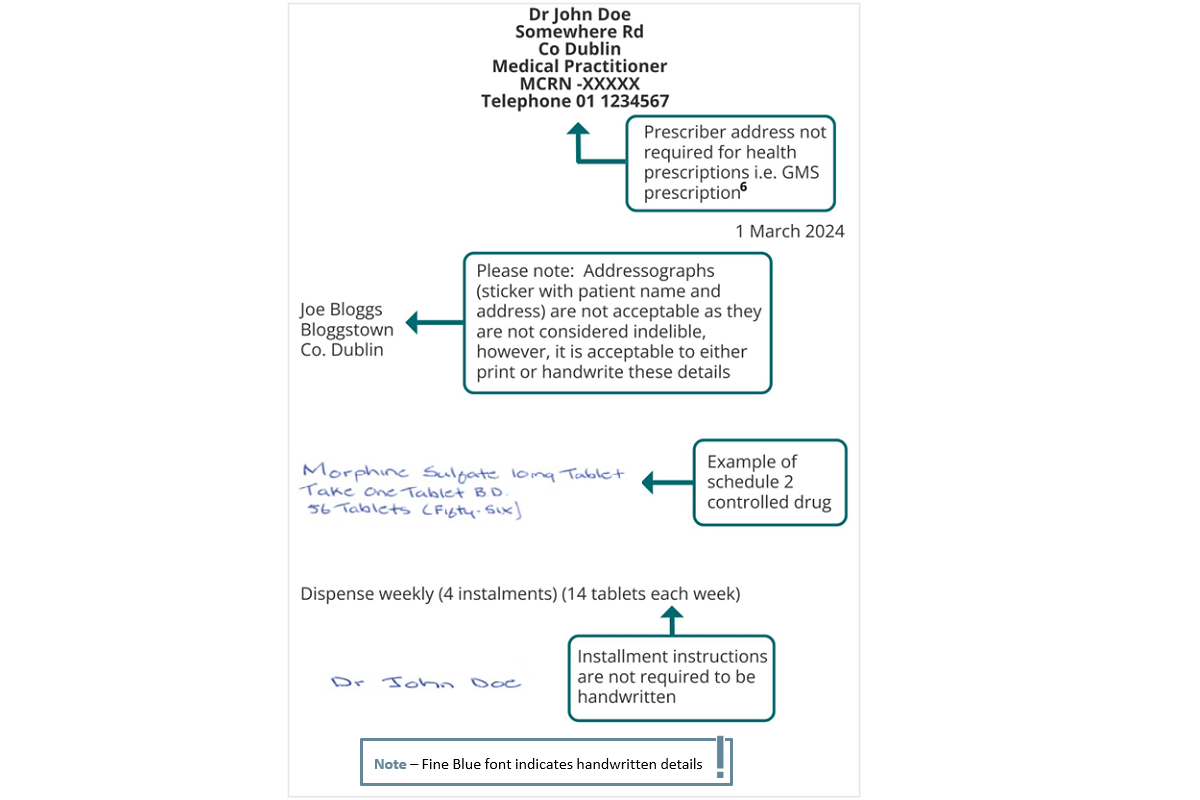

Example of Compliant Prescription for Schedule 2 and 3 CDs
Note: This example refers to a paper-based prescription for a schedule 2 and 3 controlled drug. Where schedule 2 and 3 controlled drug prescriptions are transferred via Healthmail it is not necessary for the particulars indicated below to be in the prescribers own handwriting. Prescriptions transferred via Healthmail do not need to include the prescriber’s signature in ink in so far as they can be traced electronically and securely back to the issuing practitioner.


Example of Compliant Prescription for Schedule 2 and 3 CDs
Note: This example refers to a paper-based prescription for a schedule 4 part 1 controlled drug. Where schedule 4 part 1 controlled drug prescriptions are transferred via the Healthmail, it is not necessary to include the prescriber’s signature in ink in so far as the prescription can be traced electronically and securely back to the issuing practitioner.
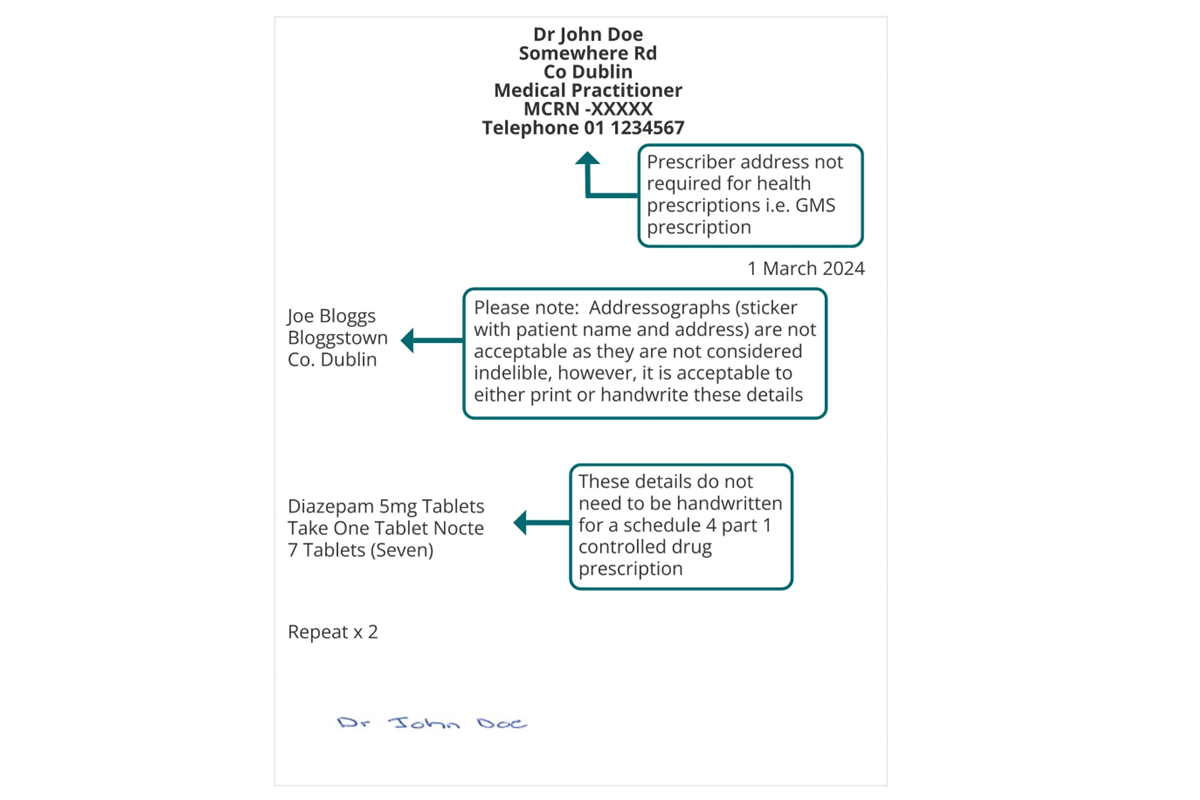

Example of Compliant Prescription Schedule 4 Part 1 CD
Example of a Compliant Requisition for Schedule 2, 3 and 4 Part 1 CDs


Example of a Compliant Requisition for Schedule 2, 3 and 4 Part 1 CDs
Note: This example refers to a paper-based GMS prescription for a schedule 4 part 1 controlled drug. Where schedule 4 part 1 controlled drug prescriptions are transferred via the national electronic prescription transfer system, it is not necessary to include the prescribers signature in ink in so far as the prescription can be traced electronically and securely back to the issuing practitioner.


Example of GMS Repeat Prescription for a Schedule 4 Part 1 CD
Note: This example refers to a paper-based opioid substitution prescription for Methadone. Where opioid substitution prescriptions are transferred via Healthmail, it is not necessary to include the prescriber’s signature in ink in so far as the prescription can be traced electronically and securely back to the issuing practitioner. All prescriptions for opioid substitution, whether paper based or transmitted through Healthmail, must be prescribed using the standard opioid substitution prescription form.
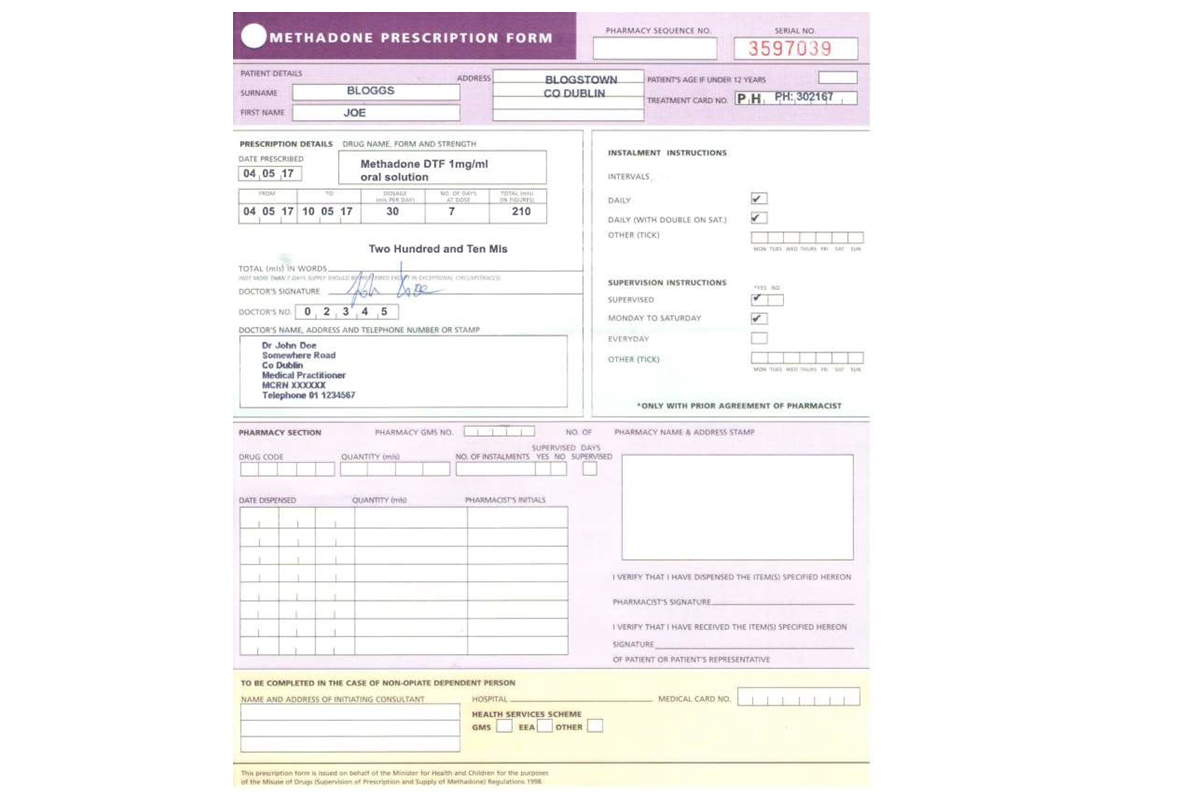

Example of Opioid Substitution Prescription
Click on the image gallery below to see the steps.


Step 1: Access HPRA Home (www.HPRA_.ie)
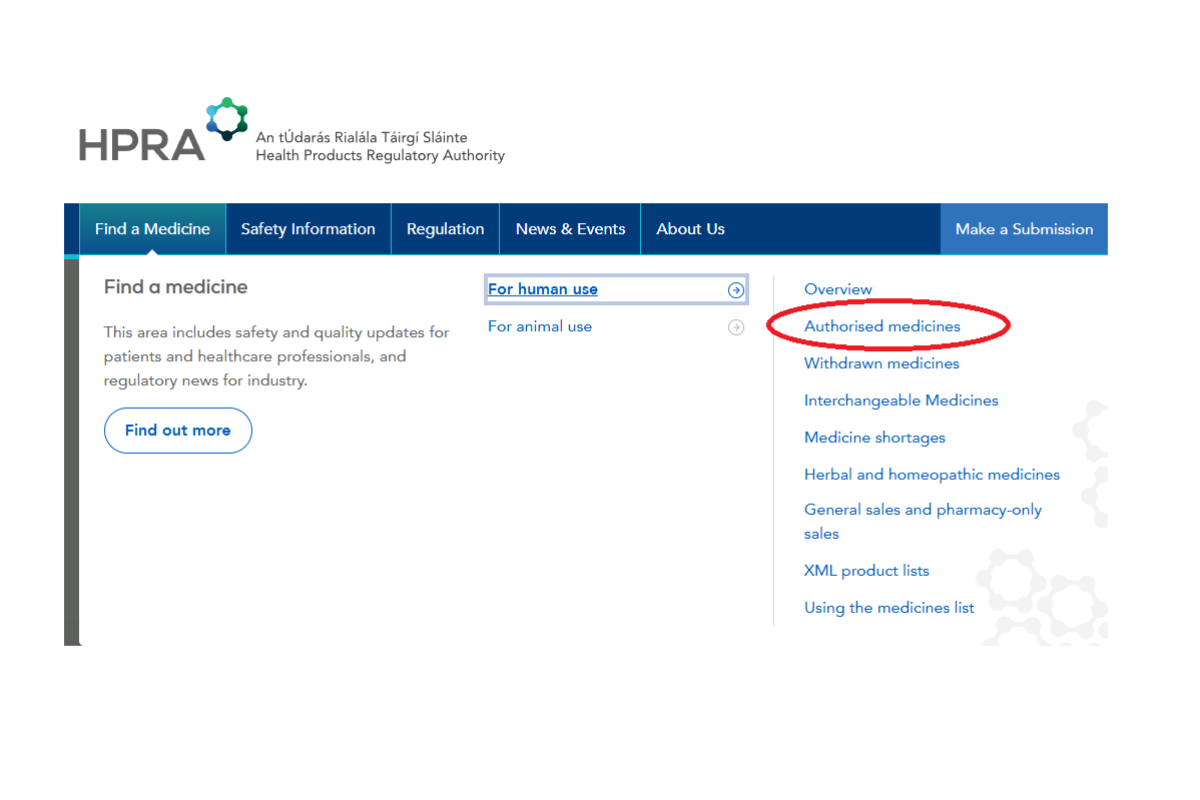

Step 2: Select medicines for human use


Step 3: Enter product name and select product
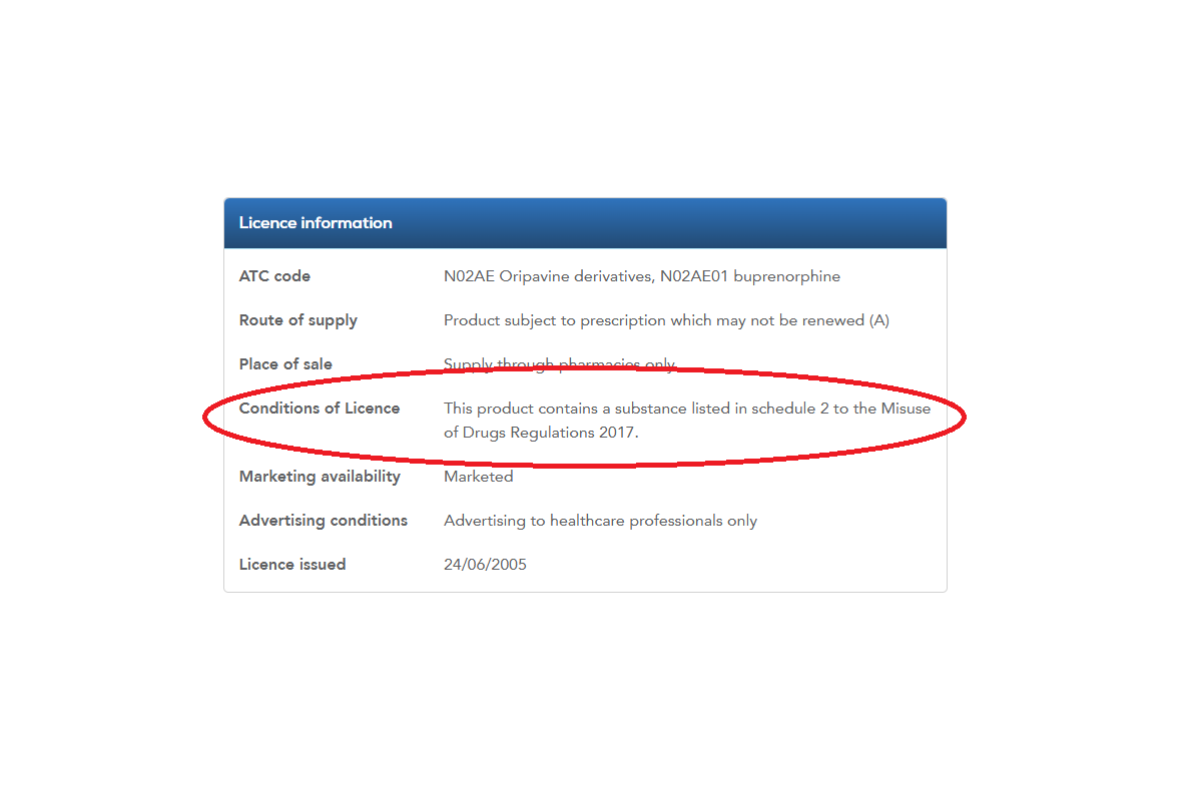

Step 4- Scroll down to ‘Licence information’ and ‘Conditions of Licence’ for details on CD Classification
Table 1 – Requirements for Paper and Healthmail Prescriptions for CD2, CD3 and CD4 part 1
* All stocks of Schedule 2 and 3 controlled drugs in the pharmacy must be stored in the CD safe in line with legislative requirements pertaining to safe custody. All receipts and supplies of Schedule 2 controlled drugs must be recorded in the controlled drug register in accordance with legislative requirements pertaining to controlled drug record keeping.
| Legal requirements | Schedule 2 and 3* | Schedule 4 part 1 |
| Must be written in ink/indelible | Paper: Yes (addressograph sticker not acceptable) Healthmail: Can be issued electronically via Healthmail. Pharmacy must print a copy. | Paper: Yes (addressograph sticker not acceptable) Healthmail: Can be issued electronically via Healthmail. Pharmacy must print a copy. |
| Include Full Name (including first name) of practitioner | Yes | Yes |
| Include Practitioner’s Registration Type and Number | Yes | Yes |
| State the Date of issue | Yes | Yes |
| Must be dispensed within 14 days of date of issue | Yes | No |
| Cannot be dispensed before date of issue stated on prescription | Yes | Yes |
| Must be signed by the prescriber in his/hers usual signature |
Paper: Yes Healthmail: Not necessary |
Paper: Yes Healthmail: Not necessary |
| State the Address of Practitioner | Yes, except for Health prescriptions | Yes, except for Health prescriptions |
| Practitioner must be registered in Ireland with an address within the state | Yes | Yes |
| Must include Practitioner’s Telephone number | Yes | Yes |
| Must include Name (including first name) and address of the patient | Yes | Yes |
| Must state the name of controlled drug |
Paper: Yes, and must be handwritten Healthmail: Yes, but does not need to be handwritten |
Yes, but does not need to be handwritten |
| Must state the dose, form, and strength of controlled drug |
Paper: Yes, and must be handwritten Healthmail: Yes, but does not need to be handwritten |
Yes, but does not need to be handwritten |
| Must state Total Quantity in words and figures |
Paper: Yes, and must be handwritten Healthmail: Yes, but does not need to be handwritten |
Yes, but does not need to be handwritten |
| Repeats allowed | No | Yes |
| Emergency supply permitted | Yes | Yes |
- Department of Health Guidance on the Medical Cannabis Access Programme is available online.
- Medicinal Products (Prescription and Control of Supply) (Amendment) Regulations 202
- Regulation 7(11) of Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended)
- Regulation 8 of the Medicinal Products (Prescription and Control of Supply) Regulations (as amended)
- Misuse of Drugs Acts 1977 to 2016 and the Misuse of Drugs Regulations 2017 (as amended)
- A Guide to Professional Conduct and Ethics for Registered Medical Practitioners
(9th Edition, 2023) page 38, paragraph 35.1 - PSI Code of Conduct- Professional Principles, Standards and Ethics for Pharmacists, 2019, page 2
- Regulation 16 (1)(f) of the Misuse of Drugs Regulations 2017 (as amended)
- Misuse of Drugs (Safe Custody) Regulations 1982 (as amended)
- Regulation 19 of the Misuse of Drugs Regulations 2017 (as amended)
- Regulation 15 of the Misuse of Drugs Regulations 2017 (as amended)
- Section 43 (8) Medical Practitioners Act 2007 and Regulation 15(2)(b) of the Misuse of Drugs Regulations 2017 (as amended)
- Specific handwriting exemptions provided for Methadone and Schedule 4 Part1 prescriptions, Regulation 15(4) of the Misuse of Drugs Regulations 2017 (as amended)
- Regulation 8(3) and 8(4) of the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended)
- Regulation 14(2) of the Misuse of Drugs Regulations 2017 (as amended)
- Regulation 15 (2)(d) of the Misuse of Drugs Regulations 2017

