Emergency Medicines Training
Training for pharmacists for the supply and administration of emergency medicines
Pharmacists who have completed the required training, are permitted to administer five medicines for the purpose of saving life or reducing severe distress in certain emergency situations:
- anaphylaxis (adrenaline),
- asthma attack (salbutamol inhaler),
- hypoglycaemia (glucagon injection),
- angina attack (glyceryl trinitrate aerosol),
- opioid overdose (naloxone).
What training do I need to complete?
The training programmes you need to complete will depend on what services you wish to provide. A diagram of the training programmes is set out below. This is known as a ‘modular’ system of training. Some of the training programmes are the same as those required to supply and administer vaccinations. If you have competed training in these programmes previously, there is information on vaccination services training requirements to assist in determining whether you need to complete the training again.
The training programmes for the specific emergency medicines you wish to administer can then be completed. This is illustrated in the diagram below.
Diagram illustrating pharmacist training requirements for the supply and administration of vaccines and emergency medicines
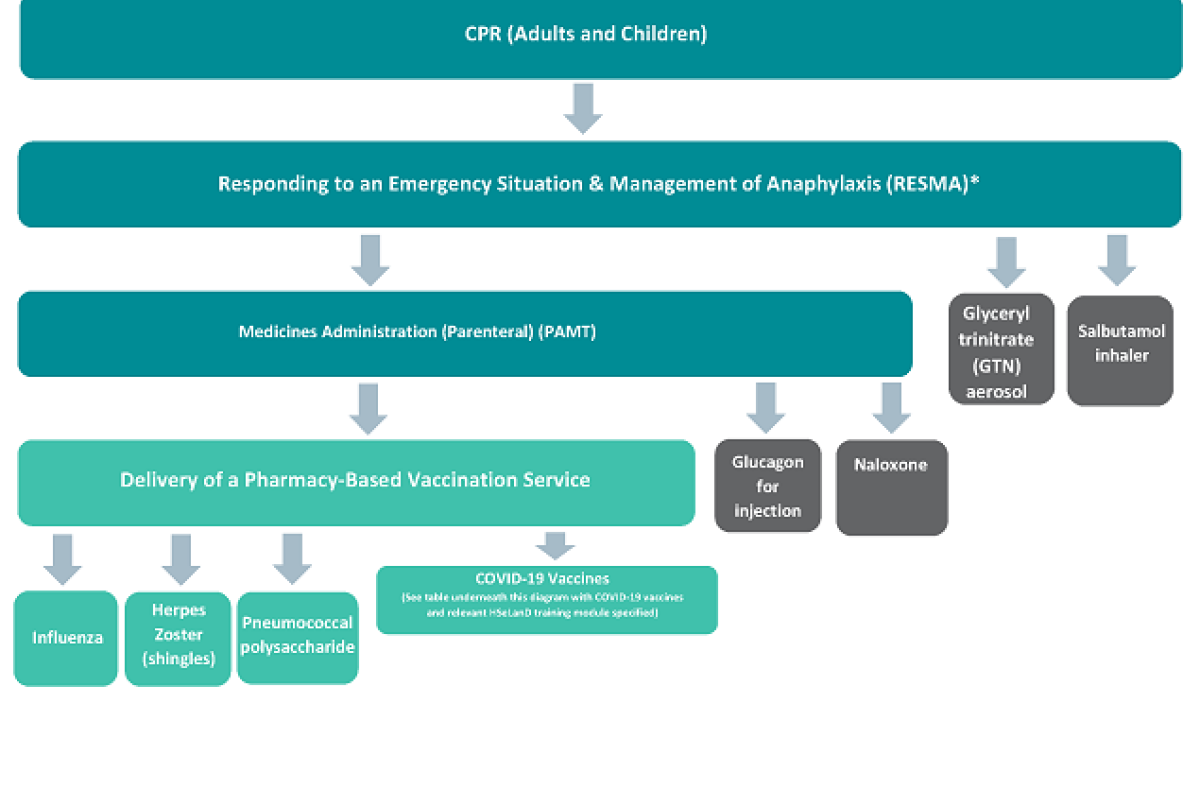
What do I need to do each year?
You should review the training requirements for the delivery of the service(s) you wish to provide each year. You should check that your training in each training programme is up-to-date. The self-assessment and self-declaration form can assist you with reflection on your skills and competency on delivering vaccination and emergency medicine services.
How can I access training and how long is it valid for?
Information on how to access the training in relation to CPR, RESMA and PAMT and the validity of the training is available here.
Information on how to access the training and the validity of the training for each of the emergency medicines is set out in the table below.
| Emergency Medicine | Validity of Training and Where to access |
|---|---|
| Glyceryl Trinitrate (GTN) Spray |
Available through IIOP Valid for 2 years |
| Salbutamol Inhaler |
Available through IIOP Valid for 2 years |
| Glucagon |
Available through IIOP Valid for 2 years |
| Naloxone | Pharmacists are required to undertake the HSeLanD online module entitled ‘Opioid Overdose Awareness and Naloxone Administration Training (Module 1)’, in addition to maintaining up-to date training in: - CPR - Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) - Medicines Administration (Parenteral) (PAMT) This means that pharmacists can build on their existing skills they have developed through the delivery of vaccinations, and no longer have to undertake specific face-to-face training to supply and administer Naloxone in emergencies. Face-to-face training (Module 2) is also available through the HSE, should pharmacists wish to complete it, though this is not a mandatory requirement. |
In addition to completing the above training pathway, pharmacists should ensure that they are familiar with the most recent versions of both the NIAC and NIO national guidance documents on management of anaphylaxis. Pharmacists should be aware of updates to relevant national guidance and adapt their practices to reflect the most up to date information.
| Notice on management of Anaphylaxis |
|---|
|
*The National Immunisation Advisory Committee (NIAC) made significant changes to the Anaphylaxis Chapter of the Immunisation Guidelines for Ireland in June 2022, which stated that “Adrenaline auto-injectors are not recommended as first line treatment by health professionals for the immediate management of anaphylaxis or suspected anaphylaxis following vaccination unless they are the only source of adrenaline available, as they may not allow IM delivery of an age appropriate dose”. However, if only an Adrenaline (Epinephrine) auto-injector is available, or if you are only trained and competent to administer an Adrenaline (Epinephrine) auto-injector, this should be used. |
If you have any questions about the training requirements, you can email cpd@psi.ie.
Useful resources
- PSI Report on the Review of Vaccination and Emergency Medicines Training Requirements for Pharmacists in 2025
- National Immunisation Advisory Committee (NIAC) Immunisation Guidelines for Ireland
- Anaphylaxis Chapter Immunisation Guidelines for Ireland
- Read the legislation which allows for these services.

