Vaccinations Training
Training for pharmacists for the supply and administration of vaccinations
Pharmacists who have completed the required training, are permitted to supply and administer the following vaccines:
- seasonal influenza vaccine,
- pneumococcal polysaccharide vaccine
- herpes zoster (shingles) vaccine
- COVID-19 vaccines
Comprehensive information for pharmacists on the training required to supply and administer certain vaccines and/or emergency medicines is set out below. You will also find it useful to reference our guidance and other resources in support of the provision of vaccination services.
What training do I need to complete?
The training programmes you will have to complete will depend on what services you wish to provide. You will receive a certificate of completion once you complete each training programme.
In order to supply and administer any vaccine, pharmacists must first undertake training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
Pharmacists are also required to have up to date knowledge on any vaccine which is administered and can meet the training requirements in this regard by:
- completing the specific training modules where available, and/or
- through review of, or having up-to-date knowledge in the relevant National Immunisation guidelines for Ireland, the Summary of Product Characteristics (SPC) for the vaccine and HSE guidance (where applicable).
Diagram illustrating pharmacist training requirements for the supply and administration of vaccines and emergency medicines
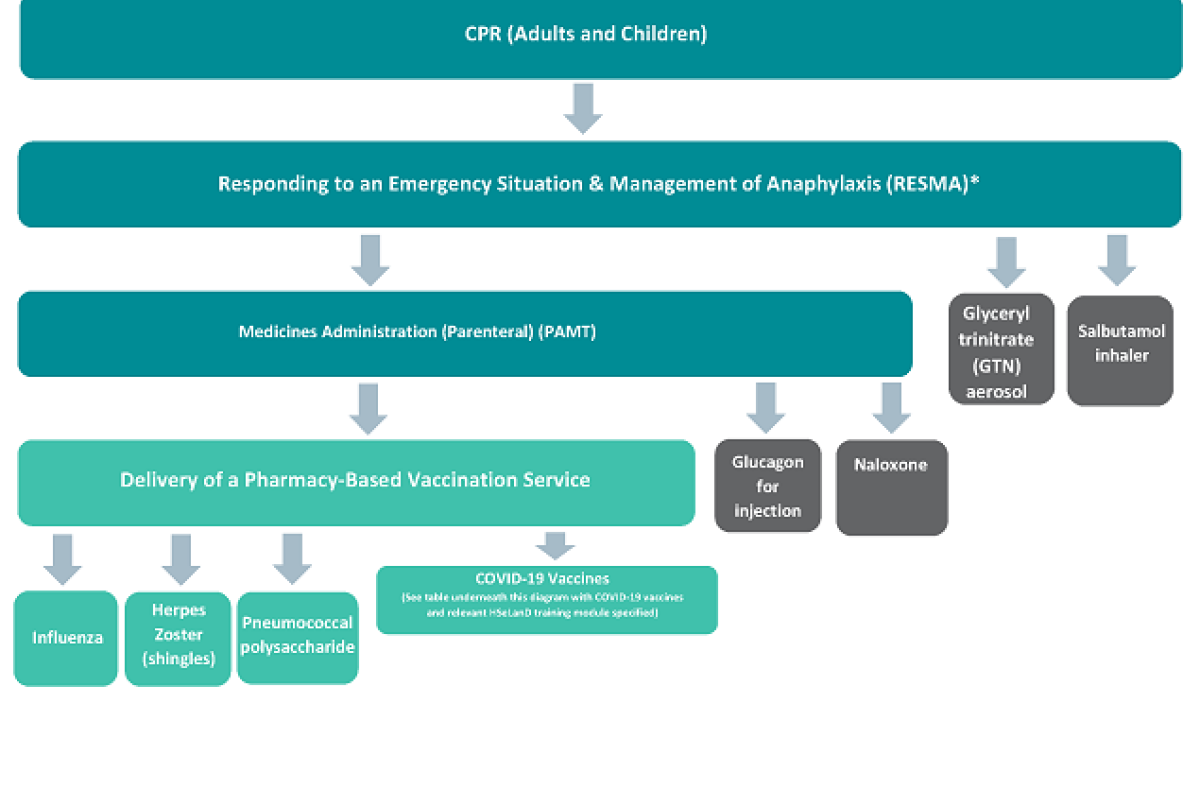
In addition to completing the above training pathway, pharmacists should ensure that they are familiar with the most recent national guidance on immunisation and the management of anaphylaxis, as contained within the Immunisation Guidelines for Ireland from the National Immunisation Advisory Committee (NIAC) and Immunisation Bulletins from the National Immunisation Office. Pharmacists should be aware of updates to relevant national guidance and adapt their practices to reflect the most up to date information.
What do I need to do each year?
You should review the training requirements for the delivery of the service(s) you wish to provide each year. You should check that your training in each training programme is up-to-date. Use the self-assessment and self-declaration form, to assist you with reflection on your skills and competency on delivering vaccination and emergency medicine services.
How can I access training and how long is it valid for?
The table below lists the individual training programmes and where you can access the training related to each training programme.
| Training Programme | Validity of training and where to access |
|---|---|
| CPR course for adults and children |
Details of training providers are available on the IIOP website. Training is valid for 2 years (or as stated by the training provider) |
|
Medicines Administration (Parenteral) (PAMT) training programme (*there is a fee associated with this training programme) |
Available as a blended programme through Hibernian Healthcare. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver the associated medicines or vaccination service. The PSI requires that this training programme is repeated in circumstances where a pharmacist has had a two-year break in vaccination practice or longer. |
| Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) training programme |
Available through the IIOP (developed by Hibernian Healthcare) Training is valid for two years. |
| Delivery of a Pharmacy-based Vaccination Service Training Programme | Available through the IIOP. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver a vaccination service. |
| Training for specific vaccines |
Available either through the IIOP (influenza, pneumococcal and herpes zoster (shingles) vaccines), or in the case of the COVID-19 vaccines through HSeLanD. • completing the specific training modules where available, and/or • through review of, or having up-to-date knowledge in the relevant National Immunisation guidelines for Ireland, the Summary of Product Characteristics (SPC) for the vaccine and HSE guidance (where applicable) The PSI recommends pharmacists to complete the online training programmes for the specific vaccines, where available, but this is not a requirement. |
| For training programmes available through HSeLanD, the HSE has developed this guide for community pharmacists to access HSeLanD training. | |
| Notice on management of Anaphylaxis |
|---|
| *The National Immunisation Advisory Committee (NIAC) made significant changes to the Anaphylaxis Chapter of the Immunisation Guidelines for Ireland in June 2022, which stated that “Adrenaline auto-injectors are not recommended as first line treatment by health professionals for the immediate management of anaphylaxis or suspected anaphylaxis following vaccination unless they are the only source of adrenaline available, as they may not allow IM delivery of an age appropriate dose”. In the absence of any other National Guidelines for the immediate management of anaphylaxis in the community, PSI would consider it best practice for a pharmacist to administer Adrenaline (Epinephrine) intramuscularly from an ampoule, in all emergency circumstances (where indicated), in accordance with NIAC guidelines. This would require the pharmacist to have valid training in: • CPR, • RESMA, and • PAMT However, if only an Adrenaline (Epinephrine) auto-injector is available, or if you are only trained and competent to administer an Adrenaline (Epinephrine) auto-injector, this should be used. Pharmacists should use their expert knowledge, skills and professional judgement to administer Adrenaline (Epinephrine) in line with National guidance in accordance with the product readily available to them, which they are trained and competent to administer. |
Useful resources
- National Immunisation Advisory Committee (NIAC) Immunisation Guidelines for Ireland
- National Immunisation Office (NIO) Immunisation Bulletins
- HSE COVID-19 Vaccine Information for Health Professionals
- Read the legislation which allows for these services.
- PSI Report on the Review of Vaccination and Emergency Medicines Training Requirements for Pharmacists in 2025




